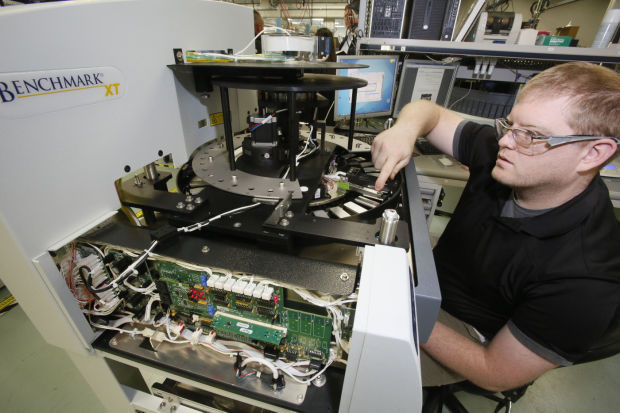
With a major manufacturing award in hand, Oro Valley-based Ventana Medical Systems is looking to the future with the development of advanced technology for digital diagnostics.
In November, Ventana was awarded the Association for Manufacturing Excellence (AME) 2014 Manufacturing Excellence Award, for demonstrating best practices in methodology, creativity and innovation.
Part of the Roche Group, Ventana develops tissue-based cancer diagnostic solutions. Research and development, manufacturing, marketing, business development and other operations are located at its Oro Valley headquarters.
More than 10,000 Ventana systems have been installed worldwide, each of which was assembled in Oro Valley before being shipped to its final destination.
Himanshu Parikh, vice president of manufacturing operations at Ventana, said an on-site audit for the manufacturing award affirmed the value of the company’s lean manufacturing process and its “embedded, continuous improvement culture.”
Parikh explained that Ventana’s instruments are assembled individually, start to finish, before the assembly of another system starts. All of the necessary parts come packed in a box that is inventoried before assembly begins, ensuring that everything is present. The system moves down the assembly line from station to station until complete and then is calibrated and tested before being shipped to its purchaser.
Ventana also manufactures all of the reagent chemicals used to create test reactions, which feature a magnetic lock-and-key system so that only Ventana products can be used in the company’s instruments.
The company makes about two million reagent dispensers annually, according to Parikh.
“Ventana is a very special cancer diagnostics company because we focus on pathology diagnostics,” said Dr. Eric Walk, Ventana’s chief medical officer. “We create tests that are meant for pathologists to use in their practice as they diagnose cancer and establish the prognosis, and now in these days of personalized health care, the companion diagnosis specific to the cancer.”
Ventana was founded in 1985 by University of Arizona pathologist Dr. Thomas Grogan, based on his idea of an instrument that would automate the process of staining and preparing tissue samples on slides for microscopic analysis.
The company had grown to 650 employees when it was acquired by Swiss drug giant Roche AG for $3.4 billion in 2008. It has since expanded its campus and now employs about 1,100 people.
Walk said Ventana has proven its products can eliminate medical errors and contamination and is now working to extend its diagnostic capabilities through digital technology.
“The pathologist has everything they need to make a diagnosis,” he said. “It used to be where the process ended, but now we’re getting into the future to enable the pathologist to use digital technology to make a diagnosis.”
Tucson Medical Center’s lab has been using all-Ventana systems for two years, but had already had components prior to the update.
John Allen, TMC’s director of lab services said that they chose Ventana’s systems because patient safety is a big issue for them. According to Allen, the products are very reliable and of great value to TMC and healthcare in general.
“These are times when healthcare needs to be very efficient and very effective,” Allen said. “That means not having to retest samples and deal with errors. This technology helps tremendously.”
Beyond an ongoing effort to improve its manufacturing processes and current products, Ventana is looking to make major inroads in the future market for personalized healthcare and digital diagnosis systems.
DIGITAL DIAGNOSIS
With a solid place in the diagnostic market, Ventana’s newest project is a slide scanner that creates a digital image and a set of software applications that allow pathologists to view and analyze slides on a monitor.
The company acquired the digital imaging technology from a company in California and has since launched its own scanner to enter the market of the developing field of digital diagnosis.
“Slides are still needed, but the image can be viewed digitally,” said Jim Fitzgibbon, Ventana’s senior manager of business development and marketing. “ An image can be magnified, annotated and measured all on a digital platform.”
Fitzgibbon explained that certain cancers, especially breast cancer, are diagnosed by counting markers to determine the percent of positivity for a specific protein.
The program uses an algorithm cleared by the U.S. Food and Drug Administration to count and analyze the cells.
“We still think it’s the human pathologist that needs to decide the case,” Walk said. “They use this still as a tool, it doesn’t get directly reported to the patient. The pathologist has to look at the result and sign off on it.”
Digitizing tissue-sample slides also has implications for telemedicine, or distance medicine, which involves sharing diagnostic and other medical information via telecommunications.
“Another application of this technology is for telepathology,” Walk said. “We’ve heard of some cases with international applications where you don’t have a pathologist in one area but you have the technical capabilities of processing the slide, they can send the image to a pathologist.”
Today’s standard for getting a second consultation is usually to ship tissue slides with a service like FedEx to the specialist, which can be a lengthy process, Fitzgibbon said.
“Now with (this) technology, you can have that secondary consultation within the hour.”
Ventana is currently working with the FDA to collect data and conduct a trial demonstrating that primary digital diagnoses using information on the screen is safe and effective.
COMPANION DIAGNOSTICS
Ventana, which makes more than 200 cancer tests, has a strong involvement in immunotherapy, a form of personalized medicine that uses the body’s own immune system to help fight cancer.
The company is developing an array of so-called companion diagnostics that detect genetic biomarkers to determine whether a specific patient would benefit from a certain therapy.
In 2007, Ventana won FDA approval to market a companion test that can screen patients for use of the breast-cancer drug Herceptin. A test Ventana developed with Pfizer for a drug to treat non small-cell lung cancer was approved in Europe in 2012.
Ventana has announced partnerships to develop other companion tests with drugmakers including Merck, Bayer, Boehringer Ingelheim and MedImmune.
“The way we got to personalized health care is because the old way didn’t work, which was essentially trial and error,” Walk said. “Historically we know that if you give all these patients a drug, only one of them is going to benefit. Why not identify up front a person who will respond to the treatment using specific tests?”
Now, diagnostics have become an integral part of drug development, Walk said.
“Within Roche we have both parts: Roche Pharmaceuticals developing drugs and Ventana and other parts of Roche Diagnostics doing the diagnostics,” he said. In the case of companion diagnostics, these come together at the end by having the FDA approve the drug and the test.”
The company is committed to developing tests that help pathologists and oncologists better understand which patients have specific biomarkers that drive cancer. Recently, research has suggested that cancer is not necessarily specific to what organ it’s found in.
“We’re learning that genes can transcend across tumor types,” Walk said. “It might be more important to identify the gene rather than the organ in which it grows.”