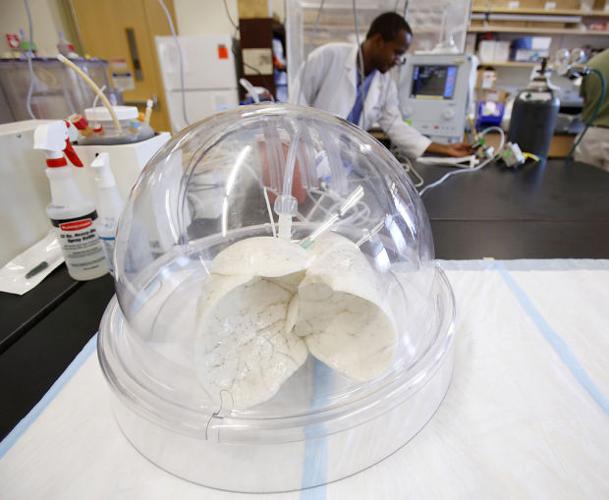
Under a clear dome inside a bustling Tucson laboratory, whitish-gray human lungs attached to a ventilator rhythmically expand and contract.
The lungs are part of a bold project at the University of Arizona that aims to forever change the landscape of transplant medicine. It would both solve an acute organ shortage and give transplant recipients the gift of a life free of powerful and damaging anti-rejection medication.
The goal is to “grow” patients’ own stem cells on a skeleton of a discarded donor organ that’s been cleaned of the donor’s living cells, and then to transplant it into the patient.
Heading up the local effort is Dr. Zain Khalpey, an Africa-born surgeon educated in England and the U.S., who is one of a handful of researchers in the world leading teams that are doing such work.
Behind Khalpey’s research is an effort to improve — and in some cases, save — the lives of transplant recipients.
Because of a shortage of organs from deceased donors, many people spend years waiting for that phone call telling them a suitable organ is available. About 18 people in the U.S. die each day waiting for an organ. Nearly 80,000 people are actively waiting.
And while it’s true that organ transplants save lives, researchers like Khalpey believe there’s room for improvement.
Now, organ recipients face chronic post-transplant health challenges from anti-rejection medication. People don’t always know that, Khalpey said. If a patient is transplanted with an organ grown from their own stem cells, ideally they would not need to use the immunosuppressant medication that transplant recipients must take for the rest of their lives.
“And that is why the drug companies don’t like me: I dislike immunosuppression,” Khalpey said.
“I’d love to implant one bio-engineered heart and one lung a day. It takes about 28 days to grow a mouse lung ... I think we’re on the cusp of something very nice.”
Reconditioned organs
Khalpey is doing other work with stem cells to help patients avoid transplants. He’s working to increase the low supply of donor organs by reconditioning lungs and hearts that would otherwise be deemed unsuitable for transplant.
“Suppose you have a 20-year-old patient who is hit by a car. Their chest is crushed. You can’t use the heart,” Khalpey said. “The heart cells have died. But you could save that heart by “decellularizing” it. I never say no to any of the poor quality human organs offered to me for research. Nothing should get wasted.”
Khalpey’s most exciting work is to literally grow organs with patients’ stem cells — a process known as organogenesis. There are two ways to do organogenesis — one is from the decellularized organ, like the lung in his lab. The other is making an organ from scratch in the laboratory. The recipe calls for a 3D printer, a “bio-ink” of proteins, fat-derived stem cells and a bioreactor to cook, or incubate it, and get it ready for transplant.
Lung transplant recipients like Debbie Ray regard organogenesis as the gold standard. It’s a way of prolonging and saving lives because it would eliminate rejection issues. Ray says it’s the anti-rejection medication that causes so many problems. She lives with weight gain, neuropathy in her feet and is at an elevated risk for skin cancer, lymphoma and osteoporosis.
“I’ve traded in a terminal illness for a bunch of little things that in the end are probably going to do me in,” said Ray, 62, who had a lung transplant at the UA Medical Center five years ago. “Now out of 10 toes I can bend two. They feel numb and sometimes it’s like walking on nails. My hair has thinned, and it has taken a toll on my kidneys,” she said.
“That seems beautiful and exciting for the future. It’s a wonderful possibility,” said Kris Patterson, a spokesperson for Donor Network of Arizona. “Right now the Donor Network of Arizona is focusing on the present and making sure those in need right now are getting help.”
While he estimates a five to 10-year timeline on organogenesis, Khalpey has several more imminent projects that he expects will help reduce the wait times and health risks organ transplant recipients now endure.
Stem-cell injections
The first is helping patients to avoid a transplant. He’ll directly inject their failing organ with stem cells in an effort to repair or regenerate it. He’s getting ready to try it on patients. One of those patients, Meg Pietsch, received a donor heart six years ago when she was 47 years old. She’s now in chronic organ rejection and spends about $8,000 per year on immunosuppressant medications. In order to avoid another transplant, she’s hoping that Khalpey’s stem-cell therapy will heal her failing heart. The stem cells could provide a better environment for the donor heart by reducing the chronic inflammation and repairing impaired muscle function.
“She is on immunosuppression medication, which means that the metabolism and integrity of her stem cells may not be great or be different to you and me,” Khalpey said. “If that is the case, immuno-privileged stem cells that we are experimenting with in the lab are amniotic stem cells could be a potential source. These cells could have the potential to do many things.”
It’s a much more favorable option than going on the waiting list for another donor heart. The wait alone can be deadly. Pietsch spent more than two years waiting for her donor heart.
“Hopefully, it will be sooner than later,” Pietsch said of the stem-cell therapy. “What have I got to lose? Dr. Khalpey is giving me hope. If it works my body will accept the donor heart.”
Ray, who attends a weekly support group for transplant patients, knows she’s fortunate compared with many other patients. Slightly more than half of lung transplant patients are alive after five years. Some don’t survive long enough to get a transplant. Some die within the first year. Ray sees that firsthand through her work with a local group that provides support to transplant patients.
“I have pain, but I’m breathing,” Ray said. “You get the transplant, and it’s a new normal you live. I’ve put on 45 to 50 pounds.”
After talking to Khalpey and visiting his lab, Ray says she is convinced that transplant medicine is in the process of changing the quality of life for future transplant patients. Khalpey and his team have already given a intravenous stem-cell transplant to one patient who was getting stem cells to reconstruct his sternum. Using stem cells, they successfully reconstructed his chest wall. At the same time, his heart function went from 40 percent to 60 percent.
A second patient was on an intra-aortic balloon pump — a partial assist device to support his heart — and injected with 100 million stem cells, and matrix were injected into the dead heart muscle. His heart function recovered from 20 percent to 34 percent in a week.
His goal is to do similar work in human clinical trials with hearts and lungs. Patients with damaged lungs could have stem-cell therapies to salvage, recondition and regenerate new lung units, minimizing and avoiding the need for a lung transplant. This could be done on ventilated ICU patients or in the clinic when diagnosed early and by avoiding transplant, the patient frees up another organ.
Anything that reduces wait times is welcomed by those on the waiting list. Ray spent two years on oxygen while she waited for a matching pair of lungs.
“I was close to checking out,” she said. “I didn’t think it would happen.”
Two-year wait
Doctors determined Ray needed a lung transplant in 2006. She was living in Sedona at the time but moved to Tucson with her husband while she waited for a lung.
She spent those two years obsessively checking her phone, and never went more than three hours from the UA Medical Center before a suitable lung donor was found from a 22-year-old man in Phoenix who died suddenly from a blow to the head. After the transplant she needed to stay in Tucson for three months, and in that time decided to move here permanently.
Khalpey came to Tucson six months ago from clinical work in mechanical circulatory support at Columbia University in New York and in cardiothoracic surgery through Harvard Medical School at Brigham and Women’s Hospital in Boston. He’s still working with patients in clinical work as surgical director of the heart transplant and mechanical circulatory support program at the UA Medical Center — University Campus, and was recently awarded an endowed chair for research in cardiac surgery.
The endowed chair was provided as an estate gift from Tony S. Marnell in gratitude to former UA heart surgeon Dr. Jack G. Copeland, who performed Marnell’s heart transplant in 1989. The transplant resulted in 12 more years of an active life. Khalpey said Copeland’s groundbreaking work on the total artificial heart at the UA was one of the factors that attracted him to Tucson.
What also drew him to the university was the support he’s getting for his work growing and restoring organs.
3-D printing
While printing out a heart on a 3-D printer may sound like science fiction, 3-D printing of bioengineered organs has already been successfully done with bladders at the Wake Forest Institute for Regenerative Medicine in North Carolina. Very few are working on organogenesis of lungs and hearts. Among those teams are researchers at Texas Heart Hospital, Harvard University, and Columbia University.
“It’s a very small group, and we are all collaborative,” Khalpey said. “Hearts and lungs are very complicated solid organs. Lungs are the trickiest. Hearts we can do sooner.”
The breathing human lung in Khalpey’s lab was frozen in a process called cryofreezing and placed in a biofarm of more than 40 organ skeletons underneath the laboratory.
“We’ve already decellularized not just pig tissue, but discarded human organs and we’re working with 3-D bio-printing of hearts, valves, tracheas and lungs,” Khalpey said. “A lot of pieces are still missing. We are focusing on the metabolism of stem cells and their microenvironment so that they don’t die in the bioreactor.”
Tucson resident and heart transplant recipient Gary Johnson is counting on Khalpey to be successful with the bioengineered organs, especially if he were to need another heart.
“It would be a blessing if he could grow a new heart for me,” said Johnson, 57. “I would welcome no steroids, no immunosuppressants. They were a lot for me to get used to, and I was nauseated. The cost is astronomical.”
In 10 years, Khalpey hopes to be relieving the stress on the donor pool for organ transplants using stem cells via regeneration, restoration and organogenesis. He makes his predictions with caution, as he knows that, for some people, the advances are a matter of life and death.
“We will be able to have patients with heart failure on mechanical devices to partially support their failing hearts while their hearts are being regenerated following stem-cell injections so they will not need a transplant. Once the heart regenerates to near normal function we would be able to wean them off their mechanical device,” he said. “This will drastically reduce the demand on the donor pool for heart transplants”
But it’s the organogenesis that would truly revolutionize transplantation.
“This will reduce the cost of health care for transplantation and make available off-the-shelf organs tailor made for patients who would otherwise be waiting or dying while waiting for their hearts or lungs on a transplant list,” Khalpey said. “That is ideal and the eventual goal. We hope we will be able to start the regeneration and restoration trials in both organs in a few months.”
“It cannot be science fiction. Stem cells have been getting a poor reputation because of medical tourism going on out there. We have to regulate the use of stem cells and prove the science to make it a fact for our patients.”