An anti-fungal drug developed at the University of Arizona, now on a fast track for approval, could cure one of the most commonly reported infectious diseases in the state.
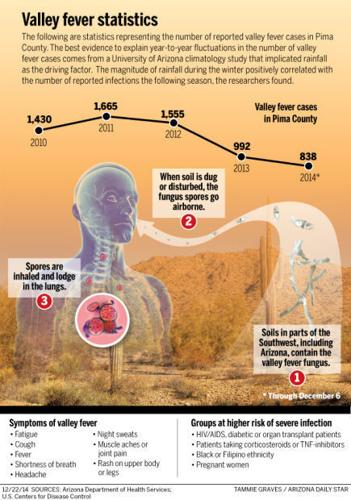
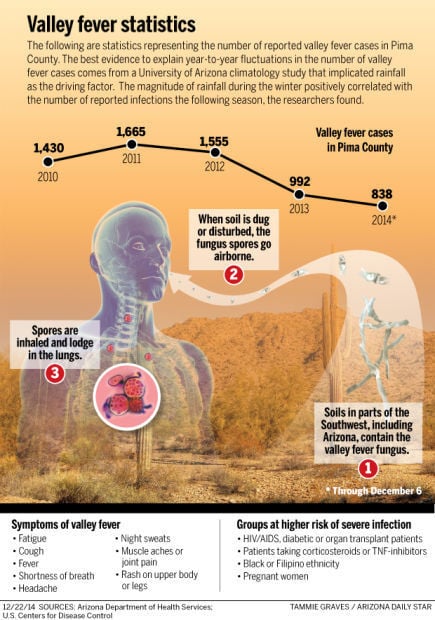
Through Dec. 6, 5,310 cases of valley fever have been reported in Arizona, including 838 in Pima County. And the numbers could still rise, since reported cases typically spike in November through December, in addition to June through August.
Valley fever (coccidioidomycosis) is caused by the coccidioides species of fungus. The fungal spores become airborne when the soil is disturbed by winds, construction, farming or other activities. In susceptible people and animals, infection occurs when a spore is inhaled.
In a small percentage of cases, the respiratory infection causes serious illness and can be lethal. The disease is unique to the Southwest, and many doctors are not familiar with it because they did their training in other parts of the country. For that reason, valley fever is frequently diagnosed long after the patient becomes ill.
The anti-fungal drug nikkomycin Z (NikZ) has been in development at the UA since 2005. Researchers have historically had trouble getting support for it because, though valley fever is common in Arizona, it is rare outside the Southwest.
The Arizona Daily Star spoke with Dr. John Galgiani, director of the UA’s Valley Fever Center for Excellence, last week about the latest development toward curing valley fever.
The following are excerpts of the interview with Galgiani. A full transcript is available on the Star’s health and wellness blog: tucson.com/news/blogs/health online.
Q: The U.S. Food and Drug Administration recently decided to designate NikZ as a “qualifying infectious disease product” — what does that mean?
A: It is a designation created by congressional legislation in 2012. QIDP designation is very important for the development of nikkomycin Z.
The drug was discovered in the 1970s, and patents surrounding its discovery are now largely expired. The QIDP designation confers five years of “market exclusivity” from the date that the drug goes on the market.
This is in addition to seven years of market exclusivity that nikkomycin Z obtained because it received “orphan drug” designation in 2006. ... Without this protection, it would be impossible to attract an investment partner to help the University of Arizona commercially develop nikkomycin Z.
QIDP also affords “fast-track” processing of applications. This means the FDA will act quickly on applications we send them.
Q: There’s a clinical trial scheduled to start for NikZ in 2015. How will that trial work?
A: There is a clinical trial scheduled for the fall of 2015. It is designed to enroll patients with very early valley fever pneumonia and start treatment early before complications have arisen. The purpose of the study is to determine if early treatment makes subjects feel better more quickly than nontreated subjects. We hope to enroll 48 subjects, 32 to receive nikkomycin Z and 16 to receive placebo.
Q: Is NikZ expected to work on dogs, since they are also affected by valley fever?
A: Yes. Their problem is very similar to humans. If nikkomycin Z was approved for human use, veterinarians would be able to also prescribe it for dogs.
Q: Is this the only valley fever drug in development?
A: Yes.
Q: Why do some people get serious cases of valley fever and others do not?
A: Most of the differences, we believe, are genetic differences, and we are now recruiting subjects to study what genetic differences may be involved.
Q: Are all Arizonans vulnerable to getting valley fever, or are some more susceptible than others?
A: There is no difference between people in the risk of infection. Most patients probably are infected because they live here. A small number of infections are linked to particular activities that expose them to unusual exposures to spores (archeology, construction, etc.).
Q: NikZ focuses on treatment and cure, but is there a way to prevent valley fever?
A: There is no way to prevent valley fever at present. We have had a long interest in finding a preventative vaccine, and recently we have identified a possible vaccine candidate that might do this and be inexpensive enough to produce.
Q: How soon before you expect it to get to market?
A: Not before three to five years. If we don’t find a partner, then the time will be significantly longer.
Q: Anything else the public should know about valley fever?
A: Two-thirds of all valley fever infections occur in Arizona, mostly between Pima and Maricopa counties. A third of all newly diagnosed pneumonias are due to the valley fever fungus.
Economic impact to Arizona from medical care and lost productivity is on the order of $300 million annually. Nationally, the Valley Fever Center for Excellence is the only such center focused exclusively on this one disease to improve our management of this major Arizona public health problem.